|
TRANSLATE THIS ARTICLE
Integral World: Exploring Theories of Everything
An independent forum for a critical discussion of the integral philosophy of Ken Wilber
  Frank Visser, graduated as a psychologist of culture and religion, founded IntegralWorld in 1997. He worked as production manager for various publishing houses and as service manager for various internet companies and lives in Amsterdam. Books: Ken Wilber: Thought as Passion (SUNY, 2003), and The Corona Conspiracy: Combatting Disinformation about the Coronavirus (Kindle, 2020). Frank Visser, graduated as a psychologist of culture and religion, founded IntegralWorld in 1997. He worked as production manager for various publishing houses and as service manager for various internet companies and lives in Amsterdam. Books: Ken Wilber: Thought as Passion (SUNY, 2003), and The Corona Conspiracy: Combatting Disinformation about the Coronavirus (Kindle, 2020). TABLE OF CONTENTS | REVIEWS
"The peer reviews [of the Corman-Drosten Review Report] overall come to the same conclusion as experts who have published their 'reviews' of the retraction request (see some examples: Beyer 1, Beyer 2, Visser, Wilson)." —Andrea Ammon, Director European Centre for Disease Prevention and Control, www.asktheeu.org, 2021-06-14.
PCR-Gate:
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| THE SARS-COV-2 VIRUS IS... | |||
|---|---|---|---|
|
Conventional Science ⇓ |
Dissident Science ⇓ |
Alternative Medicine ⇓ |
Conspiracy Theory ⇓ |
| Extremely dangerous | Mildly dangerous |
Harmless/ Beneficial |
Completely Non-existent |
| THEREFORE WHAT WE NEED IS... | |||
| Lockdown Measures | Ventilation Medication | Healthy Lifestyle | Resistance Awareness |
Who is behind this Review Report?

Patrick Savalle: ‘What virus?’
It is good to know that "investigative journalist" Patrick Savalle actually took the initiative for the Review Report, by building the team of 22 "authors".[21] This fact provides another, and somewhat contradictory and sinister context for this Review Report, regarding the very existence of viruses. How do we know viruses exist at all? He is known in the Netherlands as an AIDS-denier and anti-virus activist. He has publically challenged virologists (including Dutch virologist and advisor of the WHO Marion Koopmans, who co-authored the Corman-Drosten paper) to prove that SARS-CoV-2 actually exists, since "it has never been isolated". Ortiz Buijsse is a biochemist who has been a vocal critic of the wide use of PCR tests during the pandemic, because they are so sensitive that even the tiniest viral fragment will be detected, leading to a host of false positives—but he doesn't deny viruses exist.
Here are two videos featuring Savalle which exemplify the radicality of his views.
Curiously, neither of these two actually are part of the Borger team. Ironically, most of these team members are not virus denialists at all, for they fully agree on the existence of this particular SARS-CoV-2 virus, and the usefulness of sequencing its genome (something Savalle derides as "fun fair science"). But apparently, these various factions unite in their fight against what they consider to be disproportionate lockdown measures.
In an earlier video Savalle rhetorically asks: "Does SARS-CoV-2 actually exist?" (his implicit answer being: No, or at least: we can't be sure).
So, several things are questioned here. One, the validity of one particular PCR test, designed by the Corman-Drosten team. Two, the validity of PCR tests in general as diagnostic tools. Three, the very existence of viruses, as established by sequencing. And four, how can we be sure these viruses are the cause of a given disease like COVID-19. It is important to keep these threads apart to have a meaningful discussion.
Back to our main topic. This is extremely technical stuff, so we have to find a way to crack this nut. Pending an official reply by Eurosurveillance, let's give a breakdown of the Review Report that hopefully makes some sense to the general reader.
The Corman-Drosten Review Report
The Review Report is about 20 pages long, and gives a large number of arguments, both major and minor, listed in various ways, which makes for difficult reading. Here's a breakdown of the topics covered:
What is important when designing an RT-PCR Test
- The primers and probes
- The temperature at which all reactions take place
- The number of amplification cycles
- Molecular biological validations
- Positive and negative controls
- Standard Operational Procedure (SOP)
MINOR CONCERNS
- Incorrect nomenclature
- Incorrect direction of genetic sequence
- Missing values in a table
MAJOR CONCERNS
A) BACKGROUND
- Urgency not clear
B) METHODS & RESULTS
- Primer & Probe Design
- Erroneous primer concentrations
- Unspecified ("Wobbly") primer and probe sequences
- Erroneous GC-content
- Detection of viral genes
- Reaction temperatures
- DNA melting temperature.
- DNA amplification temperature
- Erroneous GC-contents and Tm
- No number of amplification cycles
- No molecular validations
- No positive and negative controls
- No Standard Operational Procedure (SOP)
- Consequences of the errors: false positive results.
- The Corman-Drosten paper was not peer-reviewed
- Authors as the editors
But fortunately for us the authors have summarized these at the end of their paper in 10 points:[8]
SUMMARY CATALOGUE OF ERRORS FOUND IN THE PAPER
- There exists no specified reason to use these extremely high concentrations of primers in this protocol. The described concentrations lead to increased nonspecific bindings and PCR product amplifications, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- Six unspecified wobbly positions will introduce an enormous variability in the real world laboratory implementations of this test; the confusing nonspecific description in the Corman-Drosten paper is not suitable as a Standard Operational Protocol making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- The test cannot discriminate between the whole virus and viral fragments. Therefore, the test cannot be used as a diagnostic for intact (infectious) viruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus and make inferences about the presence of an infection.
- A difference of 10° C with respect to the annealing temperature Tm for primer pair1 (RdRp_SARSr_F and RdRp_SARSr_R) also makes the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- A severe error is the omission of a Ct value at which a sample is considered positive and negative. This Ct value is also not found in follow-up submissions making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- The PCR products have not been validated at the molecular level. This fact makes the protocol useless as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- The PCR test contains neither a unique positive control to evaluate its specificity for SARS-CoV-2 nor a negative control to exclude the presence of other coronaviruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- The test design in the Corman-Drosten paper is so vague and flawed that one can go in dozens of different directions; nothing is standardized and there is no SOP. This highly questions the scientific validity of the test and makes it unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- Most likely, the Corman-Drosten paper was not peer-reviewed making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
- We find severe conflicts of interest for at least four authors, in addition to the fact that two of the authors of the Corman-Drosten paper (Christian Drosten and Chantal Reusken) are members of the editorial board of Eurosurveillance... (emphasis added)
Theoretical or Empirical Concerns?

Ian M. MacKay
Most of these "flaws" are rather technical, and we will leave them to the real PCR experts. Some have already reflected on Twitter that most of these design issues should be decided, not theoretically as if from a textbook, but practically, in a laboratory setting. It would be interesting to hear from the authors of the Corman/Drosten paper why these specific and unusual(?) design settings were chosen. For that we have to await the response from the Eurosurveillance journal editors.
Ian M. MacKay is Adjunct Associate Professor at The University of Queensland, and involved in "virology, detecting and characterizing known, new, newly identified and rare viral threats to public and environmental health". On his popular blog Virology Down Under (highly recommended!) he mentions: "I design, optimise and validate molecular tests for new and existing viruses of public health significance." Quite relevant. He recently commented on Twitter briefly on the Review Report. Here are a few highlights of his thread (with the list numbers used in the Review Report for reference):
| C/D REVIEW REPORT | COMMENTS MACKAY |
|---|---|
| B.5. No positive and negative controls | Negative controls were included. Tests for other respiratory viruses were done (all were negative). |
| 1. Incorrect nomenclature | Probably a typo |
| 2. Incorrect direction of genetic sequence | Figure title clarifies the intended use |
| 3. Missing values in a table | These are easily calculated |
| B.1.a. Erroneous primer concentrations | Not a real concern, must be tested empirically |
| B.1.b. Unspecified ("wobbly") primer and probe sequences | This is not unusual and must be tested empirically. Most probably done by design |
| B.1.c. Erroneous GC-content | Must be tested empirically |
| B.1.d. Detection of viral genes, the full genome is not covered. | This only applies to whole genome sequencing, not PCR. An accidental match with a pathogen is a non-starter, the reverse primer should also match |
| B.1.d. Primer dimers and secondary structures. | You may have a target region that is optimal so you can't move your primers. |
| B.1.d. Primer dimers and secondary structures. | Corman-Drosten don't recommend duplexing (combining) these 2 different tests. |
| B.3 No number of amplification cycles | Confusion of total cycle numbers with the threshold cycle number |
| Assumption: the world is only using Corman-Drosten's tests | Shortly after their test appeared on the @WHO site in January, it was followed by tests from China, Japan, Thailand, HK, France & US |
MacKay concludes that it is more appropriate to investigate what PCR test designs are in use in other areas of the world, instead of focussing on just one research group, "with whom at least some of the Consortium authors appear to have an unhealthy fixation." See Appendix 1 for exactly these test validation studies (provided by one of the Review Report's main authors).
That website opinion attack piece on the Corman/Drosten RT-rPCR tests has a few confused & sometimes very wrong comments.
— ??? ?. ??????, ??? ???????????? (@MackayIM) December 9, 2020
I'll preface this thread with a *presumption* I'm making - these authors haven't worked on or with this test themselves. And that matters a lot if true.
Here are some of my own reflections:
It is unclear to me how all of these 10 items would lead to false positives, as the subtitle of the Review Report claims. The whole "false positive" public discussion is greatly hampered anyways, in my opinion, by conflating two meanings: (1) a technical one, in which the test claims to have found a specific virus, but in reality reacts to a different virus, or some experimental compound (or even to itself), and (2) a clinical one, in which the test correctly finds traces of a specific virus, but in so few quantities that it doesn't pose any health problem, neither for oneself or for others. And where the percentage of technical false positives is claimed to be extremely low, the percentage of clinical false positives can be substantial, it is claimed by critics.
We have this peculiar situation that where the Corman-Drosten PCR test turns out to be slightly less sensitive (for one specific gene) compared to other tests, the opposite argument can be made that from a clinical perspective all of these PCR tests are too sensitive! So a perfectly configured (extremely sensitive and specific) PCR test in the first sense, can still generate "false positives" in the second sense—precisely because it is so sensitive: the tiniest viral fragment will be detected.
Counter-intuitively, an argument can even be made to use cheaper, less sensitive tests, especially for mass-testing and surveillance purposes (though there are some caveats):
What is more, when the Corman-Drosten test is supposedly misconfigured to detect SARS-CoV-2, one would expect false negatives to happen, not false positives. If the primers chosen are "wobbly" or ambiguous, could it not just be that this was done on purpose by the Corman-Drosten team? Why else would it have been done? I can only assume these "unspecified" primers were created to include every variation of SARS-CoV-2 that was known at the time, or any other SARS-related viruses. From the Corman-Drosten paper:
Following the rationale that SARS-CoV RNA can be used as a positive control for the entire laboratory procedure, thus obviating the need to handle 2019-nCoV RNA, we formulated the RdRp assay so that it contains two probes: a broad-range probe reacting with SARS-CoV and 2019-nCoV and an additional probe that reacts only with 2019-nCoV. By limiting dilution experiments, we confirmed that both probes, whether used individually or in combination, provided the same LOD for each target virus. The specific probe RdRP_SARSr-P2 detected only the 2019-nCoV RNA transcript but not the SARS-CoV RNA.[1]
| PROBE | SEQUENCE | COMMENT |
|---|---|---|
| RdRp_SARSr-P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ | Detects only SARS-CoV-2 |
| RdRP_SARSr-P1 | FAM-CCAGGTGGWACRTCATCMGGTGATGC-BBQ | Detects SARS-CoV-2, SARS-CoV and SARS-bat viruses |
(W=A/T, R=G/A, M=A/C). This is not a bug, it is a feature.
In an excellent discussion of primer design—yes, this can be done in a civil manner!—we even find a paragraph on the "necessity for designing degenerate primers".[9a] Degenerate primers allow for more variations than regular ones. The reason? "Designing degenerate primers and/or probes is an effective method for addressing the challenge of virus mutations... A good principle for designing highly efficient degenerate primers is to establish a limit of degeneracy of up to 64 (the number of different sequences within the primer mixture)." It is further advised to use two variants (e.g. R=A/G) instead of three (e.g. H=A/C/T). Though this is criticized by the Review Report in no uncertain terms, the Corman-Drosten test follows these guidelines. Another PCR test, from Hong Kong, followed the same principle. The reason for this is that many laboratories didn't or don't have SARS-CoV-2 samples at hand as positive controls, but they do have SARS-CoV. By enabling the test to detect SARS-CoV as well a workaround is offered for this shortage. The majority of PCR tests, though, don't use degenerate primers (the article lists 17 of these).
What is ambiguous in the Review Report list of issues, at least according to me, is that the authors seem to wobble between the extreme and unrealistic view that PCR tests as such are unsuitable as a diagnostic tool to identify a virus (see point 3), and the more specific view that the Corman-Drosten test in particular is unsuitable for that purpose, given the way it has been (mis?)configured. These are two wholly different discussions. It is plainly obvious to all medical professionals that PCR tests don't detect intact viruses but viral fragments, for the simple reason that this is how they work (and not because viruses tend to be fragmented by themselves). As to the second issue: it should be obvious that a test that was devised in such a short timespan (for whatever reason) is bound to have flaws. But these can be and have been amended as time goes by.
"PCR-Gate" is the term that is currently in use to argue that all PCR tests are "unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus", and therefore have to be abandoned. It is based on a misunderstanding of the use and limits of PCR tests. To repeat, PCR tests detect viral fragments, not viruses, but that is the whole point. That's why they can be performed relatively fast, and at low costs (especially compared to what was feasible a decade ago). So, strictly speaking having a positive PCR test does not per se mean one is infected or infectious to others. But it does provide an indication. It does prove the virus has entered your body. It depends on many other factors how that will work out in your particular case.
Yes, PCR tests have limitations, but no, these need not be fatal. Instead of suggesting we should abandon all testing, tests should be optimized.
Real science is cool..the rest is pseudoscience!
— Pieter Borger (@BorgerPieter) November 25, 2020
Optimizing Test Protocols
The mainstream media have almost completely ignored the Review Report. The authors might see confirmation here of the supposedly biased-nature of the regular press. In the Netherlands only a Christian newspaper covered it, and unexpectedly in a quite critical manner not at all anticipated by Borger (as part of a series on coronavirus conspiracies).[9] Ironically, this was the very newspaper to which Borger had contributed over the years, discussing among other topics how his Darwin-book puts Darwinism to question. Assuming he had given them a "scoop", he felt betrayed by his own newspaper. Russia- and conspiracy-friendly RT.com (Russia Today) did cover it as well.[10]

Jonas Schmidt-Chanasit
German virologist Jonas Schmidt-Chanasit stated in an interview for Welt+, that the Review Report is the usual mix of truth, opinion and falsehoods, so characteristic of disinformation about Covid-19.[11] The fact that Drosten managed to complete the test even in the absence of physical viral samples did not surprise him in the least, for it is enough to know the full genome of the virus (the sequence of which was provided by Chinese researchers, which is a fascinating story in itself[12], FV). He objected to the fact that this material was posted on a private website, and did not follow the proper channels (but then again, we are living in the Information Age—or should we say the Disinformation Age?, FV). Finally, this was only the first PCR Test, he said, and there are now many others worldwide. These sane and sensible comments substantially deflate the whole debate from theoretical to practical considerations. Indeed, many PCR tests are now in use, and research has started to evaluate their performance.
As an example, we can quote from such an evaluative study published in June 2020, comparing "Seven Different Primer-Probe Sets and One Assay Kit", where the whole Discussion section is helpful to read (and I have highlighted a few remarkable conclusions).
Known cases of COVID-19 have now exceeded 375,000 worldwide. While the number of new infections in China appears to be leveling off with fewer identified each day, the number of cases continues to rapidly increase in other nations across the world (3). In the coming days and weeks, many clinical laboratories will be developing and optimizing their own SARS-CoV-2 testing protocols. Maximizing the sensitivity and specificity of these tests is critical to efforts around the world to minimize the impact of this pandemic on global health.
A number of different primer-probe sets for use in SARS-CoV-2 detection assays and SARS-CoV-2 testing kits have been developed and are now available. As we have demonstrated here, the performance characteristics of assays using these primer-probe sets and testing kits are variable. Of the seven different primer-probe sets and one testing kit that we evaluated, all were found to be highly specific with no false-positive results observed when assays were run on samples positive for a number of other respiratory viruses. Variability was, however, observed in the sensitivities of these tests. We found assays using the CDC N2 and Corman E-gene primer-probe sets to be particularly sensitive. Assays using these sets were able to detect SARS-CoV-2 in 10 out of 10 known positive clinical samples. They were also able to reliably detect SARS-CoV-2 in a sample containing only about 6 genomic equivalents of viral RNA. In addition to our evaluation of different assays for SARS-CoV-2 detection, we also show that it is possible to significantly increase capacity for the RNA extraction step of SARS-CoV-2 testing without sacrificing sensitivity.
In summary, we report variable performance characteristics of SARS-CoV-2 detection assays using seven different primer-probe sets and one complete testing kit when applied to clinical samples. While all assays evaluated were highly specific, some, such as those using the CDC N2 and the Corman E-gene sets, were found to be more sensitive than others. These findings will provide important insights on SARS-CoV-2 detection assay design to labs that are currently working to develop their own testing methods. Our results also emphasize the importance of ongoing optimization of viral detection assays following the emergence of novel viral pathogens.[13]
In a similar validation study of PCR tests, "Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit", also from June 2020, we find this in the Abstract and Discussion sections ("RdRp Charité" refers to one of the Corman/Drosten PCR tests):
A reliable diagnostic assay is crucial to early detect new COVID-19 cases and limit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission. Since the onset of the COVID-19 pandemic, the World Health Organization has published several diagnostic molecular approaches developed by referral laboratories, including Charité (Germany), HKU (Hong Kong), China CDC (China), US CDC (United States), and Institut Pasteur, Paris (France). We aimed to compare the sensitivity and specificity of these different RT-PCR assays using SARS-CoV-2 cell culture supernatants and clinical respiratory samples. Overall, the different RT-PCR assays performed well for SARS-CoV-2 detection and were all specific except the N Charité (Germany), and N2 US CDC (United States) assays. RdRp Institut Pasteur (IP2, IP4), N China CDC, and N1 US CDC were found to be the most sensitive assays. The data presented herein are of prime importance to facilitate the equipment choice of diagnostic laboratories, as well as for the development of marketed tests...
The present study compared the performance of five RT-PCR-based methods developed by referral laboratories. N China CDC, N1 US CDC, and RdRp IP2 and IP4 were found to be the most sensitive assays on SARS-CoV-2 cell culture supernatants and clinical respiratory samples. Vogels et al. compared the performance of SARS-CoV-2 PCR assays developed by the same referral laboratories, except those from Institut Pasteur. Using RNA-spiked mock samples, they found that ORF HKU was one of the most sensitive assays [18]. Herein, ORF HKU was more sensitive than RdRp Charité but slightly less sensitive than other assays, such as N1 US CDC or N China.
Although RdRp Charité performed well for the lowest dilutions, it was nevertheless found to be less sensitive than others, a result in line with those of Vogels et al. [18]. It is worth noting that the Charité assay was the first to be published at the early stage of the pandemic [9] and has been widely used worldwide [8]. This assay was initially designed for the diagnosis of SARS-related CoVs and then optimized for SARS-CoV-2 detection [5]. Thanks to this assay, an important number of COVID-19 diagnoses were made, which contributed to limiting the spread of the outbreak. (emphasis added) [14]
Quite contrary to the authors of the Review Report, who see false positives popping up everywhere—without having demonstrated this empirically. If they are so expert, why haven't they done this?
A Matter of Tweaking and Tuning
Thus, it is all a matter of tweaking and tuning, until the best empirical results are found. Rather than bashing an early PCR test for SARS-CoV-2 it is more fruitful to focus on PCR test validation research covering the full testing landscape. Compared to the mean-spirited and rather absolutist nature of the language used throughout the Review Report, with its continuous refrain repeating ad nauseam that the Corman-Drosten PCR test is "unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus", we are now back in the real world. Concerted efforts are made all over the world, to develop and optimize SARS-CoV-2 testing protocols. As it should be, when we are faced with worldwide problems of life and death of this magnitude.

In the Washington Post of today, December 26th, it is reported that the CDC had its own problems with designing a workable PCR test for SARS-CoV-2.[15] Instead of quickly adopting the test made available by the Drosten team and the WHO, it decided to build one of its own. This caused unnecessary delays in implementation—and unnecessary deaths. As Stephen A. Morse, a retired agency microbiologists said:
It would have been prudent, he said, "to use the WHO test that was already available. At the same time, get a better understanding of the performance of the test—see if you could improve on it with a second-generation test, as opposed to trying to develop your own test, independent of what's out there."
Without tests to identify the early cases, health authorities nationwide were unable to isolate the infected and trace the rapid spread among their close contacts. Those who were asymptomatic, yet contagious, went undetected.
This is, again, sane and sensible talk, even with wisdom in hindsight, and indicates the dilemmas that had to be faced: use a less-then-perfect test now or wait for a better one (with a government that doesn't really feel the urgency of the pandemic). So much different from the Borger-team which passes harsh judgement on the Corman-Drosten test, disregarding the practical challenges the early researchers were facing and the improvements that have been made worldwide since then. The crucial question to be answered by Eurosurveillance then is, which protocol is used in Europe these days, the early Corman-Drosten one or optimized versions? Is the Review Report correct in making the assumption that the early test is still widely used?
So when all is said and done, the conclusion might just be that the original Corman/Drosten PCR protocol was sub-optimal in certain respects, but excellent in others. Given the time frame and the urgency of the early-pandemic situation this was to be expected. All over the world different protocols are now used, and some PCR tests for SARS-CoV-2 perform better than others—but most do quite well. And the PCR test remains our only way to reliably track and trace the spread of a potentially lethal new virus.
Conclusion
We could summarize the main argument of the Corman-Drosten Review Report authors as to its wider implications and agenda, as follows:
| ARGUMENT | REFUTATION |
|---|---|
| The Corman-Drosten PCR test is flawed and still widely used. | There are dozens and dozens of PCR tests, with different specs. |
| This creates a great many false positives, a "casedemic". | False positives happen only very rarely, research shows. |
| We should stop all testing. Even a perfect PCR test should not be used for diagnosis. | We need more improved tests. A PCR test can be used for diagnosis and surveillance. |
| Current lockdown measures are illegitimate and should end. | Current lockdown measures are rational and beneficial. |
In conclusion, it would be more commendable to constructively work on improving the available PCR tests, instead of arguing against the validity of one particular test, and hysterically concluding that any supposed flaws in this test cause a casedemic of false positives, which form the basis of an illegitimate global lockdown.
Unfortunately, that is precisely the agenda of the International Consortium of Scientists in Life Sciences. This agenda shades off imperceptibly into conspiracy country.
This whole affair looks a lot like a storm in a petri dish to me.
Appendix 1: SARS-COV-2 PCR TEST VALIDATION STUDIES
Kevin McKernan

Keven McKernan
One of the more expert authors of the Borger paper when it comes to PCR, Kevin McKernan[16], recently argued on Bitchute (a conspiracy channel) that the Borger paper could be merely theoretical (one of the criticisms many have brought up against it), because the lab studies had already been done.[17] "The wet work is already evident in the literature, if you read it." He refers to the following 5 scientific papers (source: PANDA: Pandemic - Data & Analytics):
- Yujin Jung et.al., "Comparative Analysis of Primer-Probe Sets for RT-qPCR of COVID-19 Causative Virus (SARS-CoV-2)", ACS Infect. Dis. 2020, 6, 9, 2513-2523. August 2020.
- Mathieu Gand, et.al., "Use of Whole Genome Sequencing Data for a First in Silico Specificity Evaluation of the RT-qPCR Assays Used for SARS-CoV-2 Detection", Int J Mol Sci. 2020 Aug; 21(15): 5585.
- Sibyle Etievant et.al., "Performance Assessment of SARS-CoV-2 PCR Assays Developed by WHO Referral Laboratories", J Clin Med. 2020 Jun; 9(6): 1871.
- Maximilian Muenchhoff et.al., "Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020", Eurosurveillance, Volume 25, Issue 24, 18 June 2020.
- Konrad, et al., "Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020", Volume 25, Issue 9, 05 March 2020.
The upshot of these articles, if I understand them correctly, is that PCR test protocols for SARS-CoV-2 should be and have been improved since the first test was published, if only because the virus has mutated in many genome locations over time and many more genomes have become available compared to the early days of the pandemic, so these mutations can be traced. PCR tests should continuously be monitored for their ability to capture these recent mutations. And yes, the early Corman-Drosten test seems to have issues in terms of sensitivity (leading to somewhat more false negatives).
"Dr. John Tal"
A corroboration of this finding can be found on an instructive (German language) YouTube channel hosted by "Dr John Tal" (alias of a biochemist with 20 years experience in biochemical and molecular-biological analytics). In one video responding to a recent Corona Ausschuss ("Corona Committee") legal session, featuring Ulrike Kämmerer, one of the main authors of the Review Report, he covers half a dozen test validation studies that have been done since the Corman-Drosten test was published.
Ein Video Kommentar zur 22. Sitzung des 'Stiftung Corona Ausschusses'
Tal concludes—quite contrary to the authors of the Review Report:
- The initial test developed by Charité [the Corman-Drosten test] showed less sensitivity in comparison with other methods with respect to one gene (RdRp).
- Neither the Charité test nor other tests showed a cross-reactivity with other germs or infections [so no false positives were observed].
- The revised Corman-Drosten test is neither better nor worse than any of the available PCR tests that are currently in use all over the world.
This means, in my understanding, that if anything can be held against the original Corman/Drosten test, it is that it leads to more false negatives, not false positives. This is widely know, and the very reason that this particular test got optimized.[18]
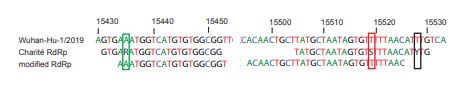
In normal language, the test may have missed some early cases of COVID-19, but it did not falsely claim people were infected by SARS-CoV-2. No false positives.
Here's another relevant video from Dr. John Tal, which recounts the rapid development and validation of the SARS-CoV-2 test by Christian Drosten:
His most recent video (7 Jan 2021) deals specifically with the Corman-Drosten Review Report:
The COVID-19 RT-qPCR Testing Landscape
A comprehensive review article from June 2020 covering both molecular and seriological tests for COVID-19 paints a drastically different picture than the one we get from the Corman-Drosten Review Report, taking the full COVID-19 testing landscape into account.[19] It turns out there are dozens and dozens of different tests available, each targeting its own mix of SARS-CoV-2 genes (ORF1a, ORF1ab, RdRP, E, N, S). A visual overview tells the story best, listing 40 different tests:
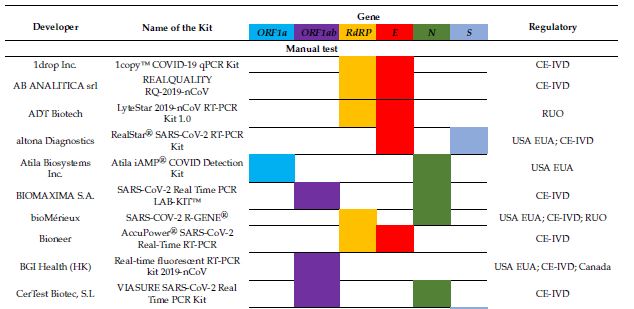
And guess what? Most if not all of these tests score very high on both sensitivity and specificity when it comes to detecting SARS-CoV-2. Again, the table tells the story:
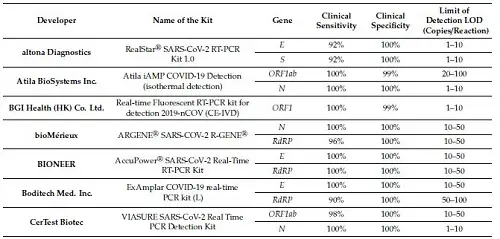
* FIND, the Foundation for Innovative New Diagnostics, is a WHO Collaborating Centre for Laboratory Strengthening and Diagnostic Technology Evaluation. Headquarters: Geneva.
APPENDIX 2: PANDEMIC DENIALISM AND VIRUS DENIALISM
This is, of course a different project than arguing that we can stop testing because all PCR tests are invalid (at least as they are used in Europe), which is the main message of the more vocal PCR critics. Let alone that there is no pandemic at all. Here's an example of how these findings are framed in the conspiracy media. Postively tested persons are here conflated with "patients", falsely concluding that only 3% of the patients had Covid-19. Of course, many of these don't need hospitalization, even if they have tested positive.
What is more, even when most tests use 35 cycles or more, the majority will turn positive (in Real Time) much earlier. The 3% relates to cases that turned postive only after 35 cycles. Which is not the same as "the PCR test produces 97% false positives"!
It?s confirmed: The pandemic is a hoax. 22 highly renowned scientists conclude that the PCR test produces 97% false positives. So out of all of the Covid patients we?ve ever seen, 97% never had Covid. STOP PCR TESTING NOW. #covid #COVID19 #stoppcrtestingnow pic.twitter.com/m7ky8eIeDn
— Karl Watchtay7 (@kvwatchtay7) December 18, 2020
Since our corona conspiracy series is about virus denialists, I could not resist quoting McKernan when he replies on Twitter to a virus denialist, in no uncertain terms.[20]
Its been thoroughly sequenced even with direct RNA sequencing on Nanopores.
— Kevin McKernan (@Kevin_McKernan) December 15, 2020
See Kim et al.https://t.co/CKk81iUkUq
And when this person questioned further, bringing up the familiar vero cells and exosomes, McKernan was even more explicit: "you have no clue what you are talking about."
Because the nanopores start at one end of the RNA and sequence all the way to other until they fall off.
— Kevin McKernan (@Kevin_McKernan) December 15, 2020
Its quite evident these are not human. If that is your argument, you are have no clue what your talking about and are easily dismissed. Get a better argument.
And when it comes to virus denialism, Kevin McKernan most definitely is not a fan:
It seems to me that Patrick Savalle, the originator-in-the-background of the Corman-Drosten Review Report[21] who continuously casts doubt on the very existence of the virus and the relevance of genomic science, has some serious homework to do.
This applies as well to one of the Consortium members, Stefano Scoglio, who is a virus denialist, as you can read in Part 25.
APPENDIX 3: "ADDENDUM": A CATALOGUE OF WET-LAB PAPERS

On January 11, 2021, the Consortium published a 48 page Addendum to their Review Report, dealing with the PCR test validation studies that have been done that purportedly show conclusively the Corman-Drosten protocol is flawed. This amounts to a list of 20 preprint or published papers in the field of external quality assessment. The main criticism the Consortium had received was that the objections raised by the Consortium were merely theoretical and not backed up by empirical research. This Addendum seeks to address that criticism. It also contains some additional considerations about the Corman-Drosten protocol and Eurosurveillance review practice.
The question as to which PCR test for SARS-CoV-2 excels and which is flawed, and where improvements have been made in the past months since the pandemic started, is a legitimate one and an interesting one. The problem I see with the Consortium's approach is that they argue that the current pandemic is a pseudo-pandemic or phantom-pandemic, due to an unfounded reliance on PCR test outcomes. This opinion is hard to reconcile with high numbers of hopital admissions and excess death rates reported in many countries (though these statistical arguments are usually disputed by lockdown skeptics as well).
This is a key conclusion of the Consortium:
In summary, the peer-reviewed literature on the defects of the Corman-Drosten primers is vast. While biases and errors may be understandable due to pandemic time constraints, those due to short-circuited peer review, conflicts of interest and regulatory capture at the WHO, should be condemned once they are identified. There is no way to maintain public trust in the scientific method and publication process when such errors affect millions of people's clinical decisions and livelihoods. (p. 35)
But then, at the end of the Addendum, there's a dramatic twist to the story:
Even if the RT-qPCR test was optimal and had theoretically sensitivity and specificity of 100%, it is medical malpractice to use RT-qPCR and other rapid tests outside the need for specific antiviral therapy in symptomatic or severely ill hospitalised patients. Interpreting positive tests as 'medical cases' without consideration of internal controls and viral Ct with clinical context, nor consideration of other viruses or diseases that cause similar symptoms as COVID-19, enables politicians to practice medicine on entire populations. This lack of clinical integration has led to problems in the past. (p. 41)
Methinks the Consortium would feel competent to create the perfect PCR test for SARS-CoV-2, but paradoxically in the end they don't care about any PCR test improvements, for in their understanding they are useless for large-scale diagnosis and surveillance of an emerging pandemic.
The whole operation by the Consortium is done in bad faith: critiquing an early PCR test for SARS-CoV-2 in the strongest terms without any intention of improving on it. The authors seem to "wobble" between two incompatible views: (1) The Corman-Drosten PCR test sucks, and (2) all PCR tests suck. But if (1), there are now many more PCR tests available for SARS-CoV-2 testing, so why focus on the very first test? And if (2), why take all this trouble at all to analyze one particular test to death if even the most perfect test would not be fit for diagnostic use?
Put differently, if even a perfect PCR test would be "unsuitable as a diagnostic test" (the phrase is repeated a dozen times in the Review Report), than the whole argument against one particular PCR test becomes a thoroughly wasted effort in futility. They should have tackled the second question: what role, if any, can PCR tests play in surveillance and diagnosis of a new virus?
As the title of the Corman-Drosten paper told us, a PCR test can be used to "detect" a virus—not more, not less.
APPENDIX 4: EUROSURVEILLANCE'S RESPONSE TO THE REPORT
On February 4, 2021, Eurosurveillance published its long-awaited response to the Corman-Drosten Review Report, after a two-month period of review by five external experts.[22]
Within one week of the receipt of the Report, and after a discussion with the editorial board members, it was decided that scientific misconduct or conflicts of interest were a non-issue:
An initial evaluation of the allegations was undertaken in collaboration with several board members, followed by a discussion at the annual editorial board meeting on 4 December 2020. This resulted in the decision that it was not necessary to publish an expression of concern with respect to allegations of scientific misconduct or fraud, and that there was no conflict of interest by the associate editors who co-authored the manuscript. It was further decided to involve external subject experts to assess the allegations related to the scientific content of the article.
This was their final conclusion:
The detailed allegations with respect to scientific flaws in the Corman et al. article were reviewed by a group of five laboratory experts. These comments were made available to the Eurosurveillance associate editors, except for those who were co-authors of the paper.
The consulted experts confirmed that the Corman et al. article was scientifically adequate for its purpose and for the limited data and material available at this early stage in the COVID-19 pandemic. Any laboratory deciding to use the primers and protocol suggested in this article would ascertain the assay for its fitness for purpose and compliance with local quality and accreditation requirements; this is what has happened worldwide since the publication of the article. With more data and evolving knowledge, laboratories have since further improved the initial method, as per usual practice.
In conclusion, after a thorough investigation in which we collected scientific advice from various sources, including several external reviewers, the editorial team—unanimously supported by its associate editors, except for those who were involved as co-authors—has decided that the criteria for a retraction of the article have not been fulfilled.
The Corman-Drosten test protocol was "adequate for its purpose" given the early days of the pandemic, and it has since then been improved, "as per usual practice."
The main authors of the Review Report were of course not amused, but had more or less expected this result, since in their opinion Eurosurveillance was an example of corrupt science. As they stated on Twitter: "For political scientists like @MarionKoopmans and @c_drosten this may be wonderful news, for real science this is another black day. Join us and free science from politics!" (Borger). "A complete joke after two months of review-time" (Bobby Malhotra) and "We need to reboot science with transparent review" (Kevin McKernan).
What specifically annoyed them was that none of the ten points of criticism was addressed in this published response (perhaps it was addressed in the anonymous reviews that were sent to the authors?). Since this is a much larger topic, we will return to this in Part 24.
NOTES
[1] Victor M Corman et. al., "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR", PMC, www.ncbi.nlm.nih.gov, (Eurosurveillance, 2020 Jan 23).
[2] Pieter Borger et. al., "Review report Corman-Drosten et al. Eurosurveillance 2020", External peer review of the RTPCR test to detect SARS-CoV-2 reveals 10 major scientific flaws at the molecular and methodological level: consequences for false positive results, November 27, 2020.
[3] See for some of these team members an initial response by Dutch skeptic: Pepijn van Erp, "Some considerations on the retraction paper for the Corman-Drosten PCR test", www.pepijnvanerp.nl, December 2, 2020. "And among the Italian signatories, we find Dr. Stefano Scoglio. A homoeopath, with a PhD in philosophy, who believes he was nominated for the Nobel Prize in Medicine and is one of those types who continue to claim that the virus has never been isolated."
[4] The fascinating story is told in Nature in this news item: Abbott, A. First past the post. Nature 423, 114 (2003). https://doi.org/10.1038/423114a
[5] Pieter Borger, Darwin Revisited: Or How to Understand Biology in the 21st Century, Scholars' Press, 2018 (a questionable publisher). A translation of the original Dutch Terug naar de oorsprong ("back to the origin", 2009). The Dutch subtitle boldly states: "Or how the new biology ends the era of Darwin". With "new biology" Borger refers to molecular and genetic biology.
[6] Pieter Borger, "A SARS-like Coronavirus was Expected, but nothing was done to be Prepared", The American Journal of Biomedical Science and Research, April 29, 2020.
[7] "Editorial note", Eurosurveillance, Volume 25, Issue 48, 3 December 2020.
[8] "Review Report", p. 15-16.
[9] Maarten Costerus, "PCR-test overleeft stortvloed aan kritiek" [PCR Test survives a tsunami of criticism], Reformatorisch Dagblad, 30 november 2020
[9a] Dandan Li, Jiawei Zhang, Jinming Li, "Primer design for quantitative real-time PCR for the emerging Coronavirus SARS-CoV-2", Theranostics 2020; 10(16):7150-7162.
[10] Peter Andrews, "Flawed paper behind Covid-19 testing faces being retracted, after scientists expose its ten fatal problems", www.rt.com, 9 Dec, 2020.
[11] Birgit Herden, "Härte kann man nicht immer einfach mit Wirksamkeit gleichsetzen", Welt, December 8, 2020. This interview is behind a paywall, a partial summary can be found here: Bernd Harder, "Der 'Corman-Drosten-Review' und der PCR-Test", GWUP, December 12, 2020.
[12] Charlie Campbell, "Exclusive: The Chinese Scientist Who Sequenced the First COVID-19 Genome Speaks Out About the Controversies Surrounding His Work", Time, August 24, 2020
[13] Arun K. Nalla et.al., "Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit", Journal of Clinical Microbiology, Volume 58, Issue 6, June 2020.
[14] Sibyle Etievant et.al., "Performance Assessment of SARS-CoV-2 PCR Assays Developed by WHO Referral Laboratories", J Clin Medv.9(6); 2020 June.
[15] David Willman, "The CDC's failed race against covid-19: A threat underestimated and a test overcomplicated", The Washington Post, December 26, 2020.
[16] "Kevin McKernan, BS Emory University, Chief Scientific Officer, founder Medical Genomics, engineered the sequencing pipeline at WIBR/MIT for the Human Genome Project, Invented and developed the SOLiD sequencer, awarded patents related to PCR, DNA Isolation and Sequencing, USA", (Author's Affiliations, "Review Report"). (It is worth mentioning that McKernan does not seem to be a conspiracy-minded person.)
[17] Bretigne Shaffer, "Kevin McKernan on the Review of the Corman-Drosten PCR Methodology that he Co-Authored", www.bitchute.com, December 9th, 2020.
[18] "Supplementary Figure S1: Alignment of the original Charité RdRp forward and reverse primers with reference sequence and modified primers." in: Maximilian Muenchhoff et.al., "Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020", Eurosurveillance, Volume 25, Issue 24, 18 June 2020.
[19] Robert Kubina and Arkadiusz Dziedzic, "Molecular and Serological Tests for COVID-19. A Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics", Diagnostics, 26 June 2020.
[20] McKernan on Twitter, December 15, 2020, responding to a virus denialist. I can't link to these tweets because McKernan has blocked me. No idea why, actually, for I only asked him if a properly designed PCR test would correctly detect the SARS-CoV-2 virus (putting to rest all talk of "The PCR off the table!" or "Where's the virus?").
[21] Description of the YouTube video "BREAKING: PCR test is off the table!": "In de BLCLBX studio bespreekt Flavio Pasquino de uitlatingen van Koopmans + de retraction paper van Borger met de initiatiefnemer ervan, Patrick Savalle." [In the BLCLBX studio, Flavio Pasquino discusses the statements of Koopmans + the retraction paper by Borger with its initiator, Patrick Savalle.]
[22] Eurosurveillance editorial team, "Response to retraction request and allegations of misconduct and scientific flaws", Volume 26, Issue 5, 04/Feb/2021.
83 Vaccine Myths from docbastard.net


